Carbon can be found in elemental form in nature as an
amorphous solid, graphite, fullerene or diamond. Overall, carbon is the
6th most abundant element in the universe and constitutes 22.85% of the human
body. Over 10 million compounds of carbon have been synthesized in the
laboratory and many thousands, if not many millions, are found in nature which
attests to the tremendous versatility of the element (a quality that lends to
the great versatility and diversity of life itself). Because Earth is
dynamic, carbon, like all elements, gets cycled through the environment and
life plays a major role in this process.
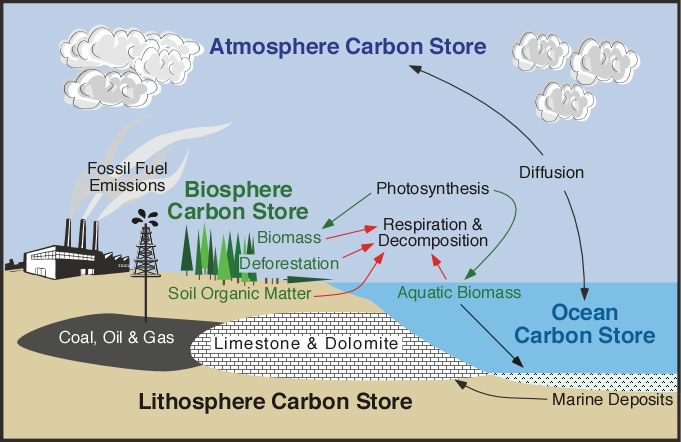
The major reservoirs of carbon vastly overshadow what is biologically cycled
and are fairly stable in ocean sediments and terrestrial rock. Fossil
fuel deposits are also a major reservoir @ 4,000 metric billion tons, but we
are adding about 9 billion metric tons of its carbon to the atmosphere through
combustion and other emissions each year. The atmosphere's carbon, mostly
in the form of CO2, is the most rapidly cycled.
Atmospheric carbon has risen from 578 billion metric tons (as of 1700) to 766
billion metric tons (as of 1999) due to human emissions. Life's total
biomass carbon accounts for, approximately, a 1,000 billion metric tons (though
it is potentially much higher depending on how many microorganisms there are on
Earth, which is hard to estimate).
Sink
|
Amount
in Billions of Metric Tons
|
Atmosphere
|
578
(as of 1700) - 766 (as of 1999)
|
Soil Organic Matter
|
1500
to 1600
|
Ocean
|
38,000
to 40,000
|
Marine Sediments and Sedimentary
Rocks
|
66,000,000
to 100,000,000
|
Terrestrial Plants
|
540
to 610
|
Fossil Fuel Deposits
|
4000
|
Estimates of Carbon Reservoirs
We'll begin the cycle with the carbon fixers or primary producers who are the plants, algae, cyanobacteria, green bacteria, and purple bacteria. The cycling activities of these organisms largely determines the potential productivity that an ecosystem can support. Their main service is to take inorganic CO2 and convert it into organic carbohydrates, lipids, proteins and nucleic acids that all other lifeforms require to get the energy and nutrition they need. They play the essential role of introducing otherwise inaccessible forms of carbon into the food web. Mostly this is done by photosynthesis, a remarkable process that converts sunlight into energy-rich molecules like sugars (Over 150 billion tons of dry organic matter is produced by photosynthesis each year!). Other methods employed by microorganisms also convert inert CO2 into organic matter such as methanogenesis (reduction of carbon dioxide to methane) and acetogenesis (reacting carbon dioxide to acetic acid). These processes require no light, but do require special molecules, metabolic pathways and enzymes. The special benefit of photosynthesis, if you appreciate breathing, is the byproduct oxygen.

Once inorganic carbon has been made into organic compounds the cycle is completed by consumers (like humans) and decomposers who, essentially, break down the organic compounds to carbon dioxide and water through respiration and fermentation. Respiration uses oxygen to breakdown molecules, while fermentation breaks down organic compounds without oxygen. Respiration is an interesting process because it is, in essence, a cell's version of an internal combustion engine minus the flame, releasing energy in a slower and more efficient manner than small explosions (like in an engine).
Decomposers play a rather under-appreciated, yet essential, role. Imagine a world where things consumed, died and bodies piled up, but never decayed. After a while Earth would be a ball of corpses with all the nutrients locked up in bodies. Thankfully decomposers exist and return lifeforms to their fundamental elements!
Microbial decomposers are also able to break down plant polymers like lignin (wood) and cellulose (the most abundant biopolymer in the world) that no one else can digest. They exist in the environment and also in the guts of termites and ruminants (cows, sheep, deer, goats, giraffes, camels, etc.) where they break down these polymers to their component sugars which animals can digest. Without this special ability plant matter would accumulate and remain, mostly, unusable.
DISCUSSION
So now that we've taken a dose of science what is the take-home-message? What can the carbon cycle teach us about life on Earth?
..............One organism's poo, is another organism's food..............
Sources:

Once inorganic carbon has been made into organic compounds the cycle is completed by consumers (like humans) and decomposers who, essentially, break down the organic compounds to carbon dioxide and water through respiration and fermentation. Respiration uses oxygen to breakdown molecules, while fermentation breaks down organic compounds without oxygen. Respiration is an interesting process because it is, in essence, a cell's version of an internal combustion engine minus the flame, releasing energy in a slower and more efficient manner than small explosions (like in an engine).
Decomposers play a rather under-appreciated, yet essential, role. Imagine a world where things consumed, died and bodies piled up, but never decayed. After a while Earth would be a ball of corpses with all the nutrients locked up in bodies. Thankfully decomposers exist and return lifeforms to their fundamental elements!
Microbial decomposers are also able to break down plant polymers like lignin (wood) and cellulose (the most abundant biopolymer in the world) that no one else can digest. They exist in the environment and also in the guts of termites and ruminants (cows, sheep, deer, goats, giraffes, camels, etc.) where they break down these polymers to their component sugars which animals can digest. Without this special ability plant matter would accumulate and remain, mostly, unusable.
DISCUSSION
So now that we've taken a dose of science what is the take-home-message? What can the carbon cycle teach us about life on Earth?
..............One organism's poo, is another organism's food..............
Perhaps a little too crude, but it captures the essence. Life is like a wheel of complementary colors. Diversity is the key because in the process of growing, digesting, regenerating, transforming and just living and dying in general, one organism requires another organism(s) that offsets their effects so that Earth can maintain some kind of balance. If animals breathed and there were no photosynthesizers, eventually, we'd all suffocate in our own carbon dioxide. Similarly, if plants converted all the carbon dioxide to oxygen, they'd suffocate too. We need each other, we need diversity, to survive because being alive means producing waste. If your environment is healthy and diverse there's bound to be some organism out there that can use your "trash" and transform it back into something usable for you. To complete the cycle we help other organisms by putting to good use their waste returning it to an accessible form for them. Think of cheese, yogurt, bread, soy sauce, pickled vegetables, alcoholic beverages, and all the other "waste products" of microorganisms that we eat as delicacies. In reality, we're just participating in the carbon cycle.
Sources:
http://www.eoearth.org/article/Carbon
http://en.wikipedia.org/wiki/Compounds_of_carbon
http://www.physicalgeography.net/fundamentals/9r.html
Microbial Ecology: Fundamentals and Applications, 4th ed by Ronald M. Atlas & Richard Bartha
-Seth Commichaux
http://en.wikipedia.org/wiki/Compounds_of_carbon
http://www.physicalgeography.net/fundamentals/9r.html
Microbial Ecology: Fundamentals and Applications, 4th ed by Ronald M. Atlas & Richard Bartha
-Seth Commichaux

No comments:
Post a Comment